The Electropolishing Process
Electropolishing is an electrochemical process that is used to remove material at a controlled rate from the surface of stainless steel. Electropolishing improves microscopic imperfections, enhances aesthetics, deburrs parts, and makes parts easier to clean. Additionally, Electropolishing provides passivation by removing free iron and contaminants resulting from manufacturing. This article will help to explain how electropolishing works and the steps of the electropolishing process.
How does electropolishing work?
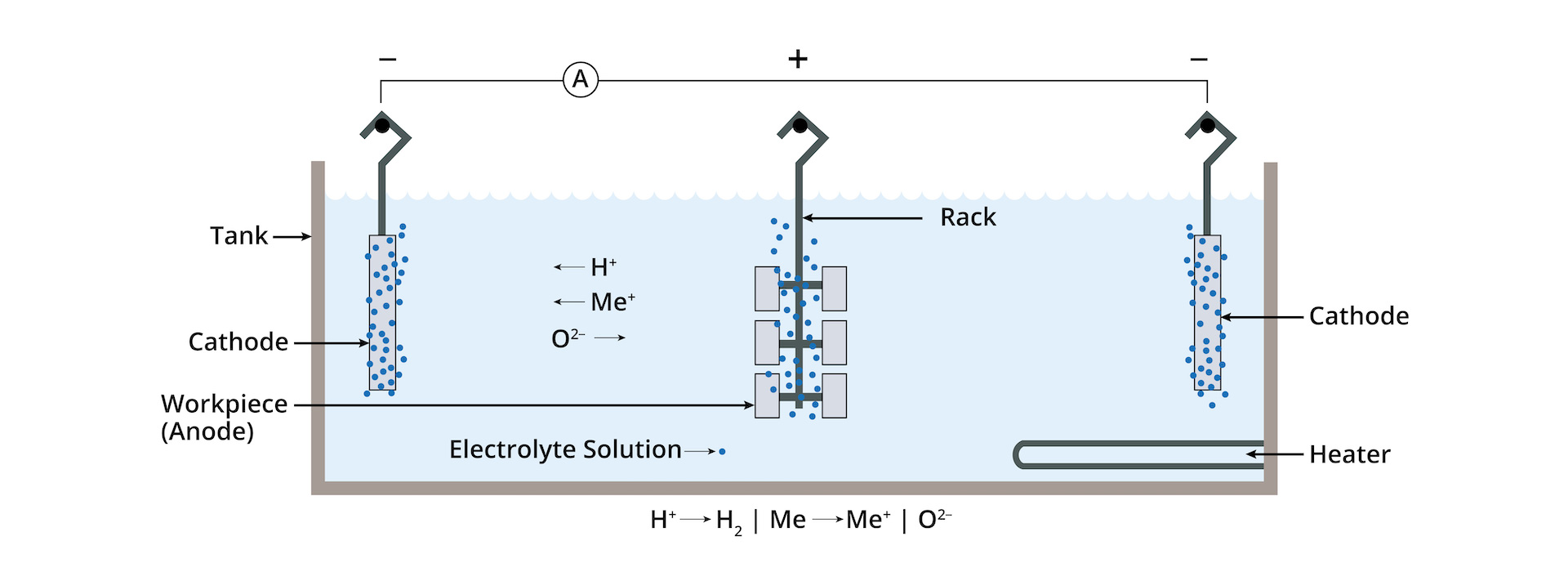
Electropolishing works by applying direct positive current to a workpiece or anode. Simultaneously, negative current is applied via cathodes. The electrolytic solution coats the workpiece and allows metal ions to be removed from a parts’ surface. As these ions are removed, the micro surface of stainless steel is improved, and small micro burrs are removed on edges and radiuses. A passive oxygen enriched layer is formed on a part’s surface.
Steps of the electropolishing process:
Pre-Treatment/Cleaning:
Upon arrival, parts undergo a pre-treatment and cleaning process to ensure the surfaces are thoroughly treated and prepared for Electropolishing. Pre-cleaning is used to remove machining oils, heat affected zones and other contaminants prior to the Electropolishing process.
Racking of parts:
Depending on the type of part and order, parts are either individually racked or combined in specially designed baskets for bulk electropolishing of small parts. Parts with higher tolerance standards, such as those used in the medical industry are individually racked for best results.
Electropolishing:
Racked parts are slowly submerged into an electropolishing bath containing an electrolyte mixture (A calibrated blend of sulfuric acid and phosphoric acid). A positive electrical current is applied to the rack which is generated from a rectifier. The electrical output (measured by amperage and voltage) is controlled remotely by a digital controller.
Post-electropolishing procedures:
The post electropolishing procedure involves a multistage rinsing process involving as many as ten rinsing stations. Parts see various acids for additional cleaning as well as clean water rinses and multi stage deionized rinsing stations as a final clean. Parts are then dried utilizing various methods depending on part size and sensitivity.
Testing:
Depending on the manufacturer’s needs, the performance of parts can be tested and analyzed through a variety of methods. Many medical parts are measured before and during processing to ensure part tolerances and thickness are maintained. NEE performs copper sulfate testing upon request to ensure parts are passive.
Final Inspection/Shipping:
Upon the completion of the electropolishing process, parts at NEE undergo a final inspection from our quality team before being approved for packaging. NEE utilizes customer packaging such as trays, cells and containers to ensure parts are well protected. Additionally, care is taken to ensure no metal-to-metal contact is made with sensitive parts.
Variables that can affect the Electropolishing Process
- Electropolishing Cycle Time
- Amperage/Voltage
- Racking/Tooling
- Temperature or Electrolyte
- Specific Gravity of Electrolyte
- Dissolved Metals in Electrolyte
- Cathode Configuration
- Tank Agitation
Important Industry Standards
Industry standards provide guidelines and best practices for Electropolishing & Passivating Stainless Steel. Without these standards, it becomes difficult for finishers and manufacturers to ensure that their parts and components satisfy and exceed finishing expectations.
Industry standards can be used as a benchmark for successful treatment and validation of Stainless Steel Electropolishing. Some of the most commonly requested industry standards used in Electropolishing and Passivation are ASTM B912 and ASTM A967.
Conclusion
It is important to choose a reputable metal finisher that understands and can maintain a consistent electropolishing process. The finisher needs to have the engineering and quality controls in place to ensure consistent and repeatable results for your stainless steel electropolishing and stainless steel passivation needs.






Electropolishing Resources
What is Electropolishing?
Electropolishing is an electrochemical and reverse plating process that removes the outer layer of skin on a metal...
The Electropolishing Process
The electropolishing process is initiated by immersing a metal part into a temperature-controlled bath of electrolyte...
How Much Material Does Electropolishing Remove?
Electropolishing, when done properly is a highly controllable process which removes as little as...
How Much Will Electropolishing Improve the Surface of my Part?
Ra and RMS are both representations of surface roughness. Ra is calculated as the roughness average of a surface’s...
What is ASTM B912?
ASTM B912 is an industry standard for the passivation of stainless steel alloys through electropolishing...
What is ASTM A967
ASTM A967 is an industry standard specification for the chemical passivation treatments for stainless...