Why does stainless steel rust?
The science behind stainless steel corrosion
Because of its resistance to corrosion, stainless steel is widely used in industries such as semiconductor, food service, marine and medical devices. However, even the most durable metals are not immune to rusting. So, why does stainless steel rust? There are several reasons why this can happen, and in this article, we will take an in-depth look at the science behind it and what manufacturers can do to avoid it.
Why stainless steel rusts…
Stainless Steels Inherent Corrosion Resistance
Stainless steel is primarily composed of iron and chromium. The chromium helps to protect the iron from corrosion by forming a protective layer that prevents the oxygen and hydrogen atoms from stripping away electrons. Therefore, stainless steel is inherently “passive” and resistant to rust and corrosion. However, if the chromium layer is damaged or weakened, often from the manufacturing process itself, then the iron becomes more susceptible to rusting and corrosion.
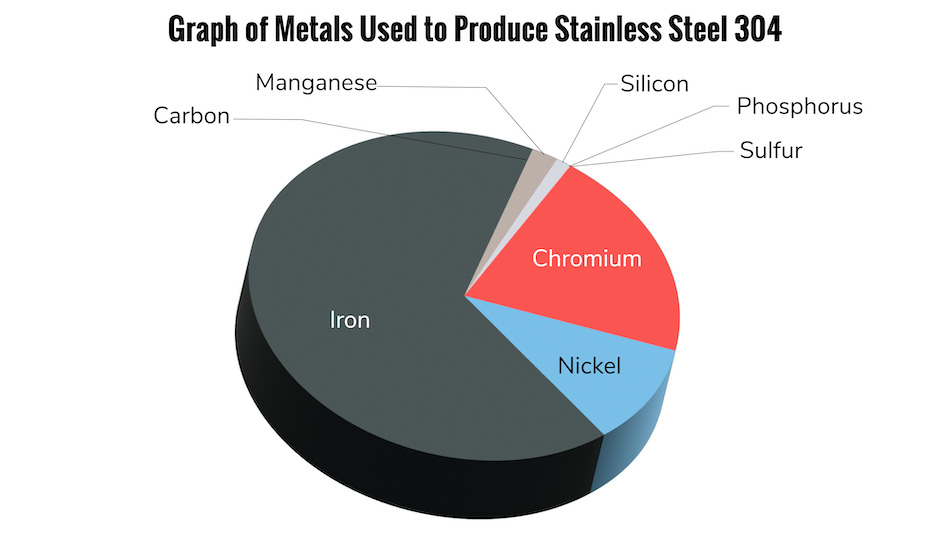
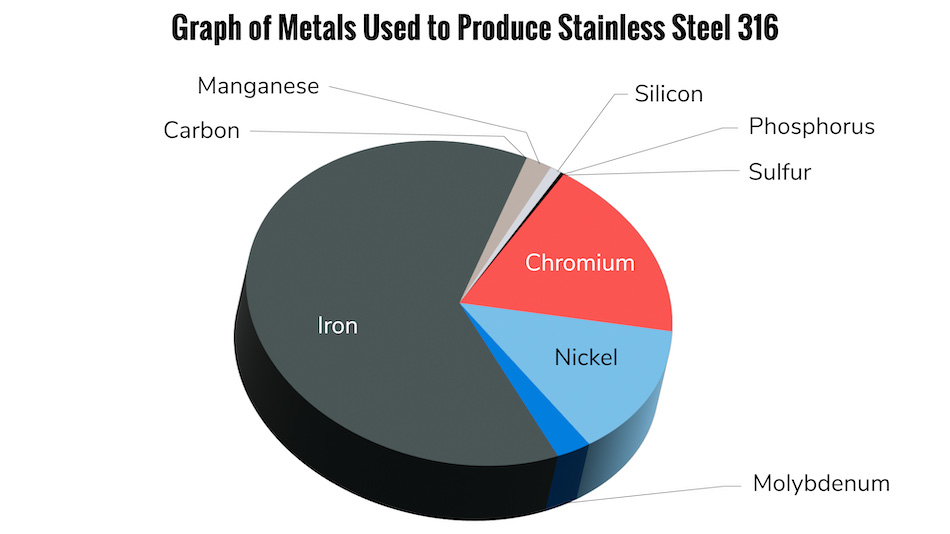
Once free iron and other impurities have been introduced into the metal through the manufacturing process, the metal becomes less resistant to corrosion and rusting. Once the metals “passive” layer has been disturbed, there are several factors that can contribute to corrosion and rusting:
Oxygen:
One of the reasons why stainless steel can rust is because of exposure to oxygen. When exposed to oxygen, stainless steel forms a thin layer of chromium oxide on its surface, which acts as a protective barrier. However, if the chromium oxide layer is damaged, oxygen atoms can penetrate the surface and react with the iron atoms in the steel, leading to rust formation.
Chloride:
Another factor that can contribute to the rusting of stainless steel is the presence of chloride ions. Chloride ions can break down the protective layer of chromium oxide on the surface of stainless steel, making it more susceptible to rust.
Water:
Water is composed of two molecules, hydrogen and oxygen. Together, these molecules form a highly reactive compound. This reaction can cause the hydrogen ions in the water to strip away electrons from the metal, causing it to corrode.
Temperature:
When stainless steel is exposed to high temperatures for an extended period, it can cause a process known as sensitization. Sensitization occurs when chromium atoms in the steel combine with carbon atoms to form chromium carbides, which depletes the steel’s ability to form a protective chromium oxide layer.
Stainless Steel Quality:
The quality of stainless steel being used can also affect its ability to resist rusting. The two most common grades of stainless steel used in manufacturing are 304 and 316. While both grades are resistant to corrosion, 316 contains additional molybdenum, which gives it greater resistance to chlorides. This makes it a better choice for applications that will be exposed to saltwater or other harsh environments that can cause rust formation.
How to prevent stainless steel rust and corrosion
Stainless steel is a versatile and durable material that is used in a variety of applications. However, even the most resilient metal can rust if not properly cared for. By understanding the factors that contribute to the rusting of stainless steel, manufacturers and product engineers can take steps to prevent it from happening. In addition, by adding processes like stainless steel electropolishing or citric acid passivation to your manufacturing process you can ensure stainless steel passivation, longer shelf life for your products, increased aesthetics, increased resistance to corrosion, improved efficacy and more.
The Benefits of Electropolishing
Examples of Manufacturing Processes that can lead to stainless steel corrosion








Electropolishing before and after examples

High Treated Machined Parts
Our small part electropolishing process will remove heat scale and contaminates on a parts’ surface while keeping material removal to a minimum (.0001-.0002” precision).

Level Floats
Electropolishing removes weld discoloration while producing a mirror finish that is corrosion resistant.

Surgical Clips
Oxide is removed through Electropolishing creating a mirror finish.

Small Electropolished Rings & Tubes
OD and ID finishing of High Purity Ring-Internal Ra 8.
Does Electropolishing Prevent Corrosion?
"Electropolishing is an electrochemical process that enhances metal alloys resistance to corrosion. Electropolishing is used to clean, deburr and passivate metals by removing impurities from the surface left behind from manufacturing processes."
Electropolishing Resources

What is Electropolishing?
Electropolishing is an electrochemical and reverse plating process that removes the outer layer of skin on a metal...

The Electropolishing Process
The electropolishing process is initiated by immersing a metal part into a temperature-controlled bath of electrolyte...

Benefits of Electropolishing
Curious about the benefits of putting your parts through the electropolishing process? Read along below where we...

How Much Material Does Electropolishing Remove?
Electropolishing, when done properly is a highly controllable process which removes as little as...

How Much Will Electropolishing Improve the Surface Finish of My Part?
Ra and RMS are both representations of surface roughness. Ra is calculated as the roughness average of a surface’s...

Electropolishing Frequently Asked Questions
Learn the difference between electropolishing and electroplating as well as how the electropolishing process works...

What is ASTM B912?
ASTM B912 is an industry standard for the passivation of stainless steel alloys through electropolishing...

What is ASTM A967?
ASTM A967 is an industry standard specification for the chemical passivation treatments for stainless...

What is ISO 13485?
ISO 13485 is a standard that applies specifically to medical devices. ISO 13485 is designed to be...
