The Passivation of Stainless Steel for Corrosion Resistance
The passivation of stainless steel is a process that helps protect the metal from corrosion and rust. A metal is considered “passive” when it shows resistance to corrosion in an environment where it would be expected to take place. There are multiple ways to achieve stainless steel passivation including nitric acid passivation, citric acid passivation and electropolishing.
Understanding the Passivation of Stainless Steel
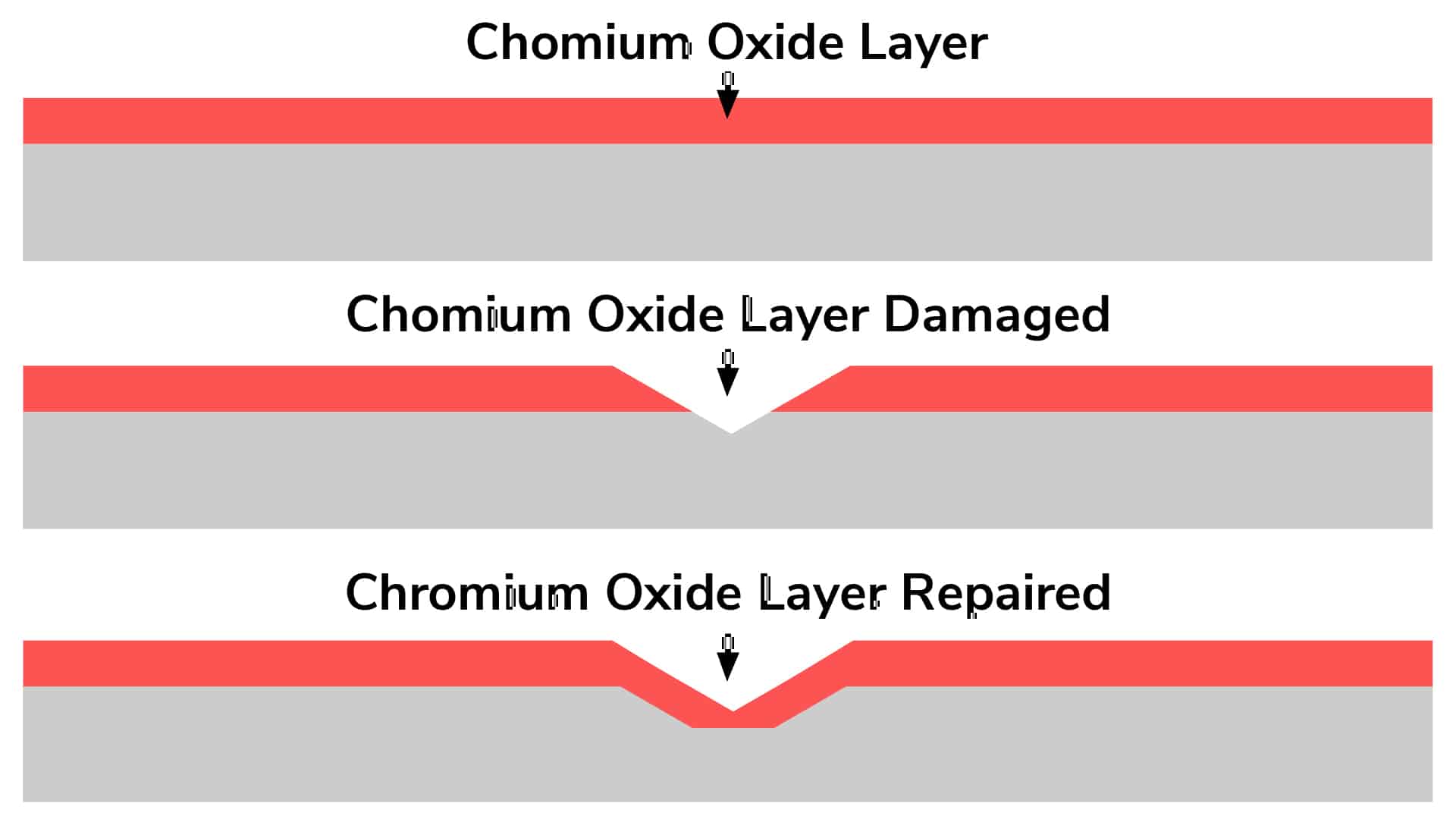
To understand the stainless steel passivation, it helps to understand why stainless steel corrodes or rusts in the first place. The chromium in stainless steel forms a thin, oxygen-enriched, passive layer on the steel’s surface. This layer makes the metal inherently resistant to corrosion. However, manufacturing processes such as rolling, cutting, machining, laser cutting, welding, and other production steps disrupt the passive layer and embed “free iron” and impurities into the surface layer of the metal. When this free iron begins to react with elements from the environment such as salt water, chlorine and many other chemicals when combined with these impurities leads to corrosion. Stainless steel passivation works by removing any impurities or contaminants on the surface of the metal and restoring the chromium oxide layer that is normally present, thereby restoring the stainless steel’s inherent corrosion resistance.
Why Passivate Stainless Steel?
Passivation is a crucial process for enhancing the natural corrosion resistance of stainless steel. Despite its inherent ability to resist rust, stainless steel can still accumulate contaminants like free iron and other impurities during fabrication or exposure to harsh environments. These contaminants can compromise its protective Chromium Oxide layer, leading to rust and corrosion. By passivating stainless steel, these surface contaminants are removed, allowing the metal to form a clean, uniform Chromium Oxide layer that reinforces its corrosion resistance.
Passivating stainless steel is especially important in industries like medical devices, aerospace, and food processing, where cleanliness and durability are paramount. The process extends the material’s lifespan, improves performance in corrosive environments, and ensures compliance with industry standards. In short, passivation maximizes the benefits of stainless steel, making it a more reliable and long-lasting choice for critical applications.
Chemical Passivation Methods:
Citric Acid Passivation
Citric acid is an environmentally friendly alternative for stainless steel passivation. This process effectively removes free iron and other surface contaminants while enhancing the formation of a chromium-rich oxide layer. Citric acid passivation is non-toxic and biodegradable, making it a safer choice for both workers and the environment.
Advantages:
- Eco-friendly and non-hazardous
- Suitable for industries with stringent health and safety regulations, such as food processing and medical devices
- Effective at removing surface iron without attacking the base metal
Common Applications:
- Medical and pharmaceutical industries
- Food and beverage processing equipment
- Aerospace and electronics components
Nitric Acid Passivation
Nitric acid passivation is the traditional method for stainless steel passivation and is highly effective at removing free iron and other impurities. Nitric acid is more aggressive than citric acid and is better suited for heavier contaminant loads or where more aggressive surface cleaning is needed. This process is commonly used in industries with high corrosion demands, such as chemical processing and marine environments.
Advantages:
- Proven track record of effectiveness
- Suitable for applications with severe corrosion resistance requirements
- More aggressive cleaning, ideal for heavily contaminated surfaces
Common Applications:
- Chemical and petrochemical industries
- Marine environments
- Heavy industrial applications
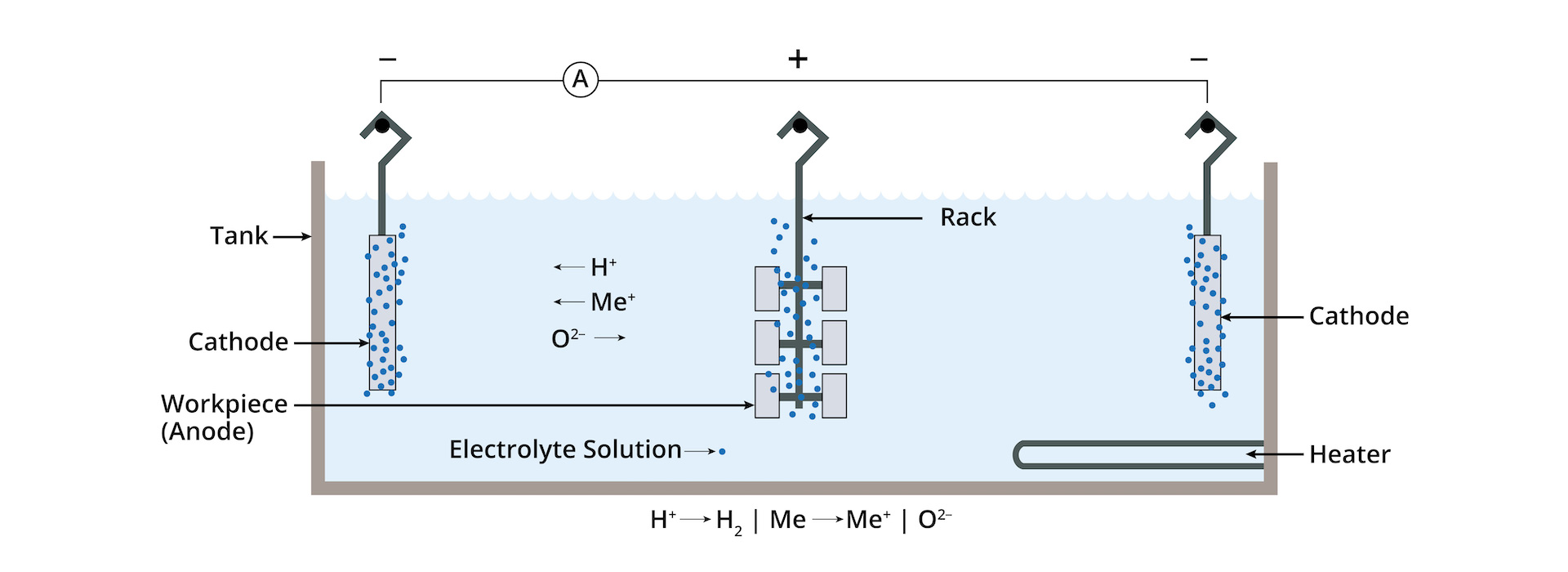
Achieving the Passivation through Electropolishing
There are multiple processes that can help to achieve passivation in stainless steel including nitric acid passivation and citric acid passivation. However, scientific studies have shown that stainless steel passivation through electropolishing is the most effective means of achieving passivation.
At NEE we agree; parts passivated through an abbreviated electropolishing cycle meet or exceed the corrosion resistance performance of parts passivated using a chemical method.
Passivation through electropolishing provides these key benefits:
The electropolishing process is compliant with ASTM B 912-02 and ASTM A967-13, “Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts.”
Passivation through electropolishing removes all free iron from the surface of the work-piece, immediately eliminating free iron corrosion potential.
Graphic Showing How Passivation Restores the Chromium Oxide Layer of Stainless Steel
A Stainless Steel Passivation Case Study: Battery Wharf
Battery Wharf serves as a prime illustration of the transformative power of electropolishing in resolving intricate corrosion challenges and extending the longevity of stainless steel in demanding conditions. With over three decades of experience, New England Electropolishing has partnered with numerous organizations grappling with comparable scenarios involving stainless steel structures and components. Don’t wait until it’s too late to safeguard your stainless steel investments.
Important Certifications for the Passivation of Stainless Steel
Certifications play a critical role in ensuring that stainless steel passivation meets industry standards for quality, safety, and performance. The most recognized certifications for passivation include ASTM A967, AMS 2700, and ISO 13485. ASTM A967 and AMS 2700 set specific requirements for the passivation process, ensuring that contaminants are effectively removed and corrosion resistance is enhanced. ISO 13485 is crucial for industries like medical device manufacturing, guaranteeing that the passivation process meets stringent regulatory standards for biocompatibility and safety. These certifications provide confidence that the passivated stainless steel will perform reliably in demanding environments.
Summary
In conclusion, passivation is an important step in the manufacturing process for ensuring that your products remain safe and reliable over time by providing an extra layer of protection from corrosive elements present in the environment. It also provides improved aesthetics and makes cleaning parts easier too! With all these benefits of electropolishing in mind, it’s clear why many product engineers utilize electropolishing to achieve stainless steel passivation. To achieve passivation in stainless steel for your products, contact the experts at New England Electropolishing today. We offer a free sample part electropolished and shipped back to you at no cost.
Passivation of Stainless Steel Resources
The Hidden Variable in Stainless Steel Performance: Surface Chemistry Stability Through Passivation
When engineers and manufacturers talk about stainless steel performance, the...
Stainless Steel Passivation in Extreme Environments: Offshore, Aerospace, and Cleanroom Applications
Stainless steel is chosen for demanding applications because of its ability to form...
Citric Acid Passivation for Additive Manufacturing (3D-Printed Stainless Steel)
Additive manufacturing, or 3D printing, has rapidly advanced from prototyping into...
Electropolishing Resources

What is Electropolishing?
Electropolishing is an electrochemical and reverse plating process that removes the outer layer of skin on a metal...

The Electropolishing Process
The electropolishing process is initiated by immersing a metal part into a temperature-controlled bath of electrolyte...

Benefits of Electropolishing
Curious about the benefits of putting your parts through the electropolishing process? Read along below where we...

How Much Material Does Electropolishing Remove?
Electropolishing, when done properly is a highly controllable process which removes as little as...

How Much Will Electropolishing Improve the Surface Finish of My Part?
Ra and RMS are both representations of surface roughness. Ra is calculated as the roughness average of a surface’s...

Electropolishing Frequently Asked Questions
Learn the difference between electropolishing and electroplating as well as how the electropolishing process works...

What is ASTM B912?
ASTM B912 is an industry standard for the passivation of stainless steel alloys through electropolishing...

What is ASTM A967?
ASTM A967 is an industry standard specification for the chemical passivation treatments for stainless...

What is ISO 13485?
ISO 13485 is a standard that applies specifically to medical devices. ISO 13485 is designed to be...